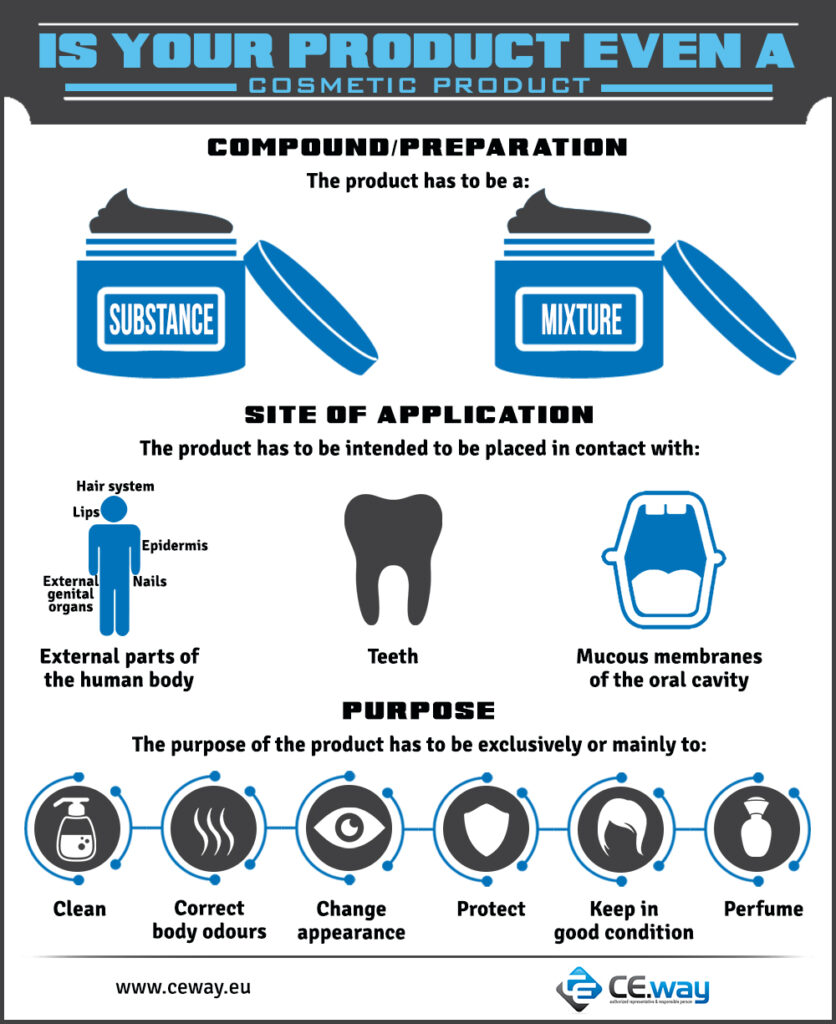
Cosmetic products definition in the Regulation 1223/2009 as follows:
| ‘Cosmetic products definition’ means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours. (EU Regulation 1223/2009, Article 2.1.a) |
The assessment of whether a product is a cosmetic product has to be made on the basis of a case-by-case assessment, taking into account all characteristics of the products. Products containing substances or preparations intended to be ingested, inhaled, injected or implanted in the human body do not come under the Cosmetic products definition.
COMPOSITION
It is not enough for the product only to be a substance or a mixture, and have the right site of application, purpose and claims, but it also has to have the correct composition. Cosmetic products should not contain certain prohibited ingredients (listed in the Regulation 1223/2009 Annex II), obey restrictions on restricted substances (restrictions are laid down in Annex III), and need to conform with the requirements connected with colorants (Annex IV), preservatives (Annex V) and UV filters (Annex VI).
BORDERLINE PRODUCTS
Special attention should be paid to the so called borderline products. The term \’borderline products\’ refers to the products where it might be difficult to classify a product into one or another product category.
A product can fall on the cosmetic/medicinal product borderline, cosmetic/biocide borderline, cosmetic/medical device borderline etc. The decision for such products is made on a case-by-case basis, taking into account all characteristics of the product, and it often depends on the claims of the product.
However, the European Commission has published a number of guidance documents to facilitate the application of Community legislation in these cases, which can be accessed here: http://ec.europa.eu/consumers/sectors/cosmetics/cosmetic-products/borderline-products/index_en.htm.
CLASSES OF COSMETIC PRODUCTS
Cosmetic products may include:
- creams, emulsions, lotions, gels and oils for the skin,
- face masks,
- tinted bases (liquids, pastes, powders),
- make-up powders,
- after-bath powders,
- hygienic powders,
- toilet soaps,
- deodorant soaps,
- perfumes, toilet waters and eau de Cologne,
- bath and shower preparations (salts, foams, oils, gels),
- depilatories,
- deodorants and antiperspirants,
- hair colorants,
- products for waving, straightening and fixing hair,
- hair-setting products,
- hair-cleansing products (lotions, powders, shampoos),
- hair-conditioning products (lotions, creams, oils),
- hairdressing products (lotions, lacquers, brilliantines),
- shaving products (creams, foams, lotions),
- make-up and products removing make-up,
- products intended for application to the lips,
- for care of the teeth and the mouth,
- products for nail care and make-up,
- products for external intimate hygiene,
- sunbathing products,
- products for tanning without sun,
- skin-whitening products,
- anti-wrinkle products
A correct cosmetic products definition is vital in order to determine which EU Directive or Regulation applies for the product.