Cosmetics animal testing
The ban of cosmetic testing on animals was already announced in the 7thAmendment of the Cosmetics Directive 76/768/EEC and since 11th March 2013 a total marketing ban of products tested on animals applies.
The animal testing prohibition is also extended to all beauty and hygiene products tested on animals outside the EU, which may not be sold inside the EU.
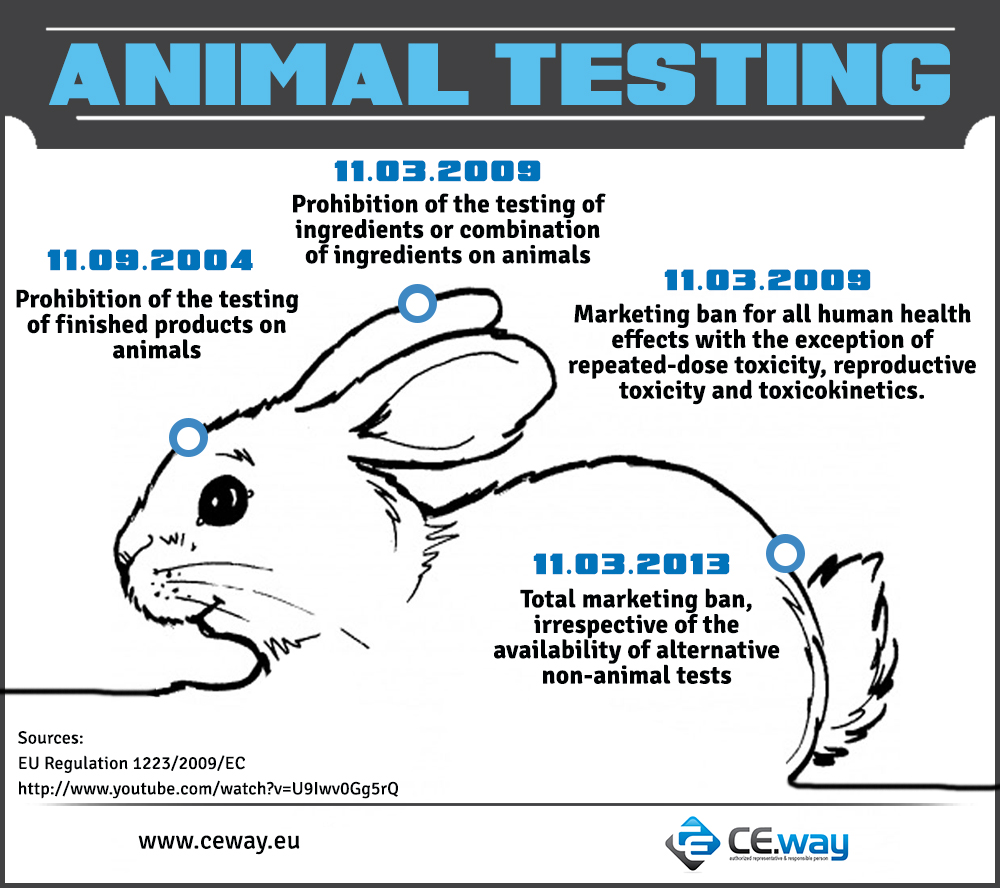
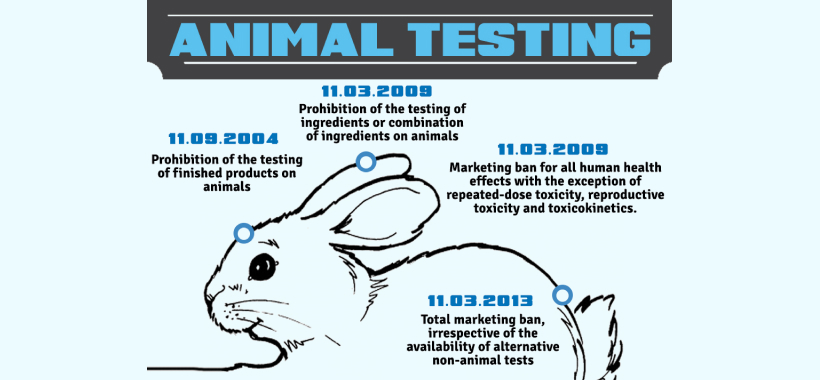
Application dates for the ban of animal testing are the following:
- 11th September 2004: prohibition of the testing of finished products on animals
- 11th March 2009: prohibition of the testing of ingredients or combination of ingredients on animals
- 11th March 2009: marketing ban (ban of sales) for all human health effects with the exception of repeated-dose toxicity, reproductive toxicity and toxicokinetics. For these specific health effects the marketing ban will apply step by step when alternative methods are validated and adopted
- 11th March 2013: total marketing ban, irrespective of the availability of alternative non-animal tests
Any tests performed on animals (of finished products or raw materials) have to be reported and included in the Cosmetic Product information File.
For a timetable of phasing out of animal testing, please follow this link to the EU Commission: http://ec.europa.eu/consumers/sectors/cosmetics/files/doc/antest/sec_2004_1210_en.pdf
For more info contact us https://ceway.eu/