EU cosmetics regulation compliant
Cosmetics manufacturers that want to start selling their cosmetic products on the EU market need to follow these 5 steps in order to be able to do so:
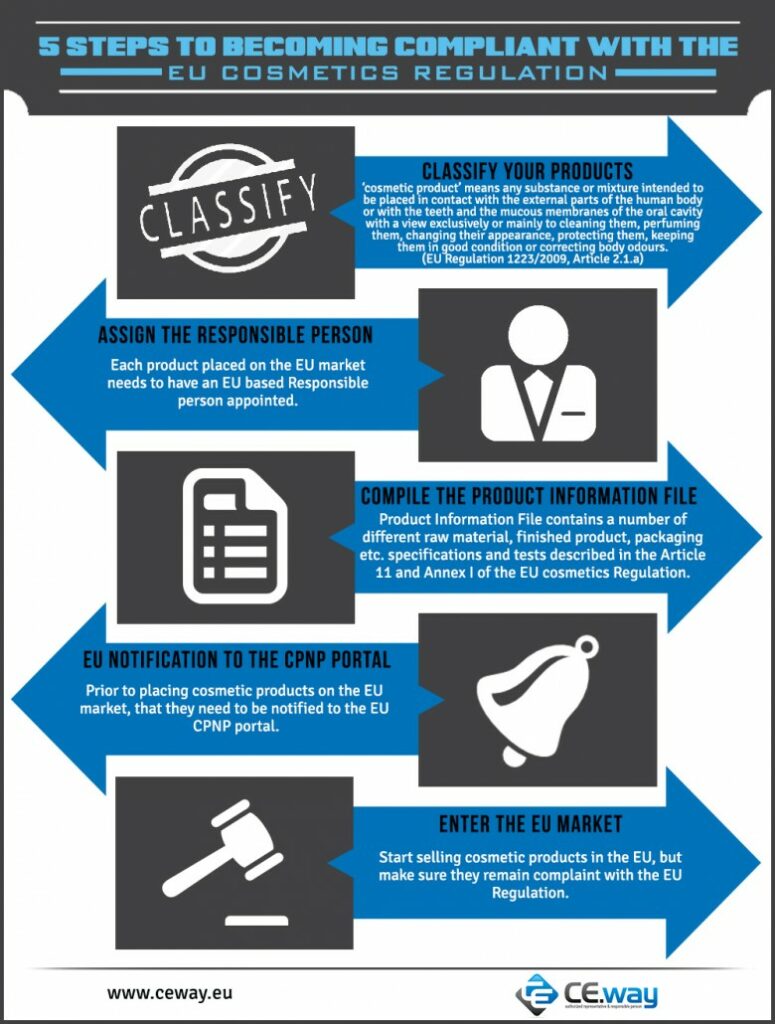
1. Classify your products
Manufacturers firstly need to determine if their products are indeed cosmetic products or not. Product classification may not be as obvious as it may seem. Certain products that would be classified as cosmetics in other parts of the world could be classified as biocides, medical devices, pharmaceuticals, toys etc. in the EU. Classification often depends on the product’s claims.
In the EU cosmetic products are defined as: ‘cosmetic product’ means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours. (EU Regulation 1223/2009, Article 2.1.a).
2. Assign the Responsible person
Each product placed on the EU market needs to have a Responsible person assigned. Responsible person needs to be based in the EU, so if the manufacturer is based outside of the EU, then he needs to appoint a company in the EU to act as the Responsible person for his products placed on the EU market. The assigned Responsible person can either be the distributor, importer, a professional Responsible person or any other third company or person. There has to be only one Responsible person assigned per product for the whole EU.
Only cosmetic products for which a legal or natural person is designated within the Community as ‘responsible person’ shall be placed on the market. (EU Regulation 1223/2009, Article 4.1).
In order to establish clear responsibilities, each cosmetic product should be linked to a responsible person established within the Community. (EU Regulation 1223/2009).
3. Compile the Product Information File
Before cosmetic products are placed on the EU market, they need to have a Product Information File (PIF) compiled. Product Information File has to be kept by the Responsible person, and it contains a number of different raw material, finished product, packaging etc. specifications and test described in the Article 11 and Annex I of the EU cosmetics regulation compliant.
4. EU notification to the CPNP portal
Responsible person has to notify the cosmetic products to the EU CPNP portal before they’re placed on the market.
Prior to placing the cosmetic product on the market the responsible person shall submit, by electronic means, the following information to the Commission… (EU Regulation 1223/2009, Article 13.1)
5. Enter the EU market and maintain your products compliant with the EU cosmetics Regulation
After these 4 steps are done, manufacturers can start selling their products on the EU market. They must keep in mind though, that their cosmetic products must always remain compliant with the EU cosmetics Regulation, and that any changes made to the product must be reflected in the Product Information File.