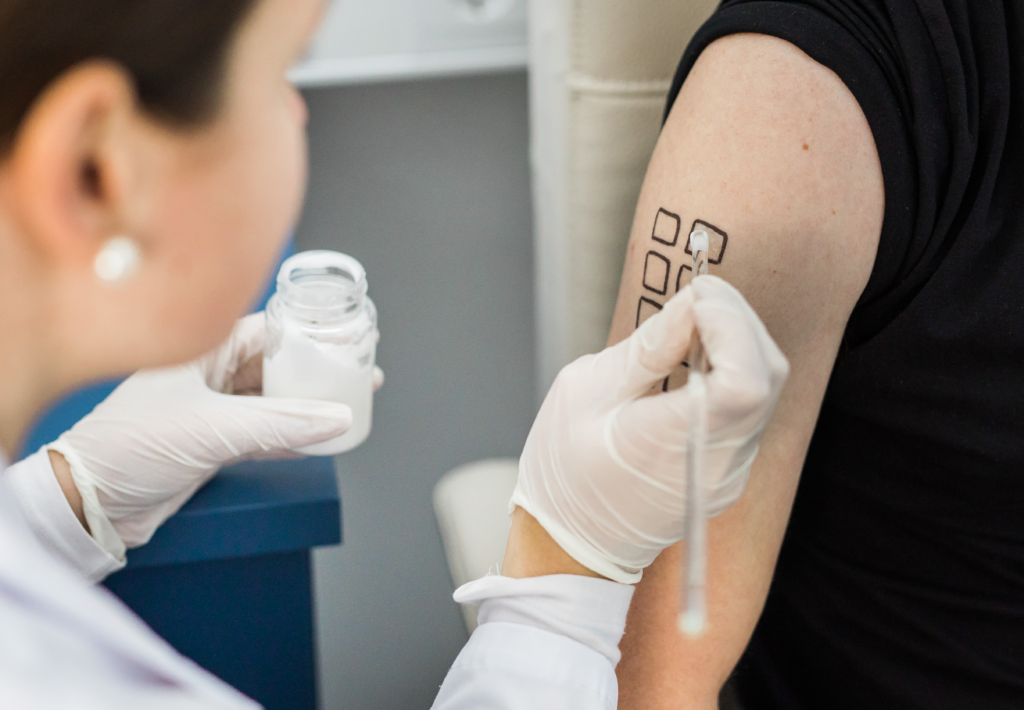Types of patch tests
Cosmetic safety testing involves a series of evaluations to assess the potential for adverse reactions and irritations that may result from the use of these products. These tests are tailored to specific areas of application, such as the skin, eyes, genitals, and oral cavity, and consider the unique needs of different user groups, including children […]