49th amendment to the IFRA code of practice
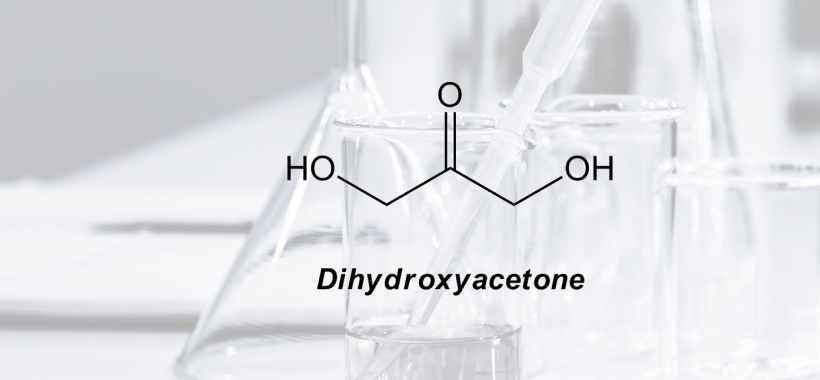
The International Fragrance Association (IFRA) published the 49th Amendment to the IFRA Code of Practice. The 49th IFRA includes 25 new standards and now covers 214 substances. Additionally, there were also some alterations to the existing standards. The two major updates include: Implementation of a revised quantitative risk assessment called QRA2, which is used to […]